Mohit Sharma
A revolutionary technique for determining the 3D shape of proteins is booming. Cryo-EM is a version of electron microscopy, which was invented in the 1930s. These microscopes use beams of electrons rather than light to form images of samples. Because the wavelength of an electron is much shorter than the wavelength of light, electron beams reveal much smaller things. In the last years, cryogenic-electron microscopy (cryo-EM) underwent the most impressive improvement compared to other techniques used in structural biology, such as X-ray crystallography and NMR
In the mid-1970s, scientists came up with the idea of freezing samples to preserve the natural structure of biological specimens and reduce damage from the electron beam, and cryo-EM was born. The technology slowly evolved, and then a few years ago took a giant leap, thanks to dramatic advances in detectors and software. In 2017 three scientists were awarded the Nobel Prize in chemistry for their roles in developing cryo-EM.
Cryo-electron microscopy (CryoEM) has superseded X-ray crystallography and NMR to emerge as a popular and effective tool for structure determination in recent times. It has become indispensable for the characterization of large macromolecular assemblies, membrane proteins, or samples that are limited, conformationally heterogeneous, and recalcitrant to crystallization. Besides, it is the only tool capable of elucidating high-resolution structures of macromolecules and biological assemblies in situ. A state-of-the-art electron microscope operable at cryo-temperature helps preserve high-resolution details of the biological sample. The structures can be determined, either in isolation via single-particle analysis (SPA) or helical reconstruction, electron diffraction (ED) or within the cellular environment via cryo-electron tomography (cryoET). This has resulted in breaking the boundaries with respect to both the size of the macromolecules/ assemblies whose structures could be determined along with the visualization of atomic details at resolutions unprecedented for cryoEM.
Today, cryo-EM generates 3-D images at nearly atomic resolution of viruses, molecules and complex biological machines inside the cell, such as the ribosomes where proteins are synthesized. By flash-freezing these tiny things in their natural environments, scientists can see how they are built and what they do in much more detail than before, stringing thousands of images together to create stop-action movies and even taking virtual “slices” through cells, much like miniature CT scans. Meanwhile, cryo-EM instruments have become easier to use and much more accessible.
Sub Techniques Within Cryo-Electron Microscopy
1. Scanning electron cryomicroscopy (cryo-SEM).
2. Cryo-correlative light and electron microscopy (cryo-CLEM).
3. Cryo-electron tomography (cryo-ET).
4. Transmission electron cryomicroscopy (cryo-TEM).
Recent applications of Cryo-EM
The novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has arisen as a global pandemic affecting the respiratory system showing acute respiratory distress syndrome (ARDS). However, there is no targeted therapeutic agent yet and due to the growing cases of infections and the rising death tolls; discovery of the possible drug is the need of the hour. In general, the study for discovering therapeutic agent for SARS-CoV-2 is largely focused on large-scale screening with fragment-based drug discovery (FBDD). With the recent advancement in cryo-electron microscopy (Cryo-EM), it has become one of the widely used tools in structural biology. It is effective in investigating the structure of numerous proteins in high-resolution and also had an intense influence on drug discovery, determining the binding reaction and regulation of known drugs as well as leading the design and development of new drug candidates. Here, we review the application of cryo-EM in a structure-based drug design (SBDD) and in silico screening of the recently acquired FBDD in SARS-CoV-2. Such insights will help deliver better understanding in the procurement of the effective remedial solution for this pandemic.
The field of Cryo-EM has gained enough popularity that only certain samples, such as viruses and ribosomes, are occasionally imaged using X-ray crystallography. Cryo-EM has now provided imaging at atomic resolution of the structural changes that occur in the p97 protein. This protein is an important target for cancer drug development as the structure and interactions of the protein are critical for cancer cell activity. Through the advanced imaging abilities of Cryo-EM, the type of p97 inhibitor binding and contact sites have been observed. This study achieved resolutions of 2.3 ångströms, with the unit ångström being the equivalent of 0.1 nanometers. Cryo-EM has the potential for further improvements to resolution with advances in detector technology and sample preparation currently under way.
Cryo-EM Facilities in India
The National Facilities supported by the Science & Engineering Research Board (SERB), an institution under the Department of Science & Technology (DST), would help explore Macromolecular Structures and Complexes” and create research knowledge base and skills for cryo-EM research in India to establish leadership in structural biology, enzymology, ligand/drug discovery. The establishment of these facilities in all directions of the country–Indian Institute of Technology, Chennai; Indian Institute of Technology, Bombay; Indian Institute of Technology, Kanpur; and Bose Institute, Kolkata would help in scaling up cryo-EM based structural biology research in different corners across the country. These centers are designated as SERB National Facility for Cryo-Electron Microscopy and will work on the identified thrust areas. They will be accessible to all researchers across the globe.
General significance
Previous structural biology techniques included X-ray crystallography and nuclear magnetic resonance spectroscopy. Both methods have had limited application because of the need for large sample sizes. X-ray crystallography also necessitates the crystallization of specimens, a difficult process that changes the environment to one that is non-physiological. Cryo-EM does not require large sample sizes or crystallization and is therefore suited to the visualization of structures at near-atomic resolution. The method also has the advantage of not chemically fixing or staining the specimen, meaning it can be studied within the native physiological environment. Moreover, without the restriction of crystals locking the sample in a static pose, structures can be flash-frozen in several conformations to allow biological mechanisms to be deduced. The cryo-EM method can be used to determine the three-dimensional structure of bio-macromolecules in near native condition at close to atomic resolution, and has the potential to reveal conformations of dynamic molecular complexes. Cryo-EM is a powerful tool for the investigation of biological macromolecular structures including analysis of their dynamics by using advanced image-processing algorithms. The method has become even more widely applicable with present-day single particle analysis and electron tomography.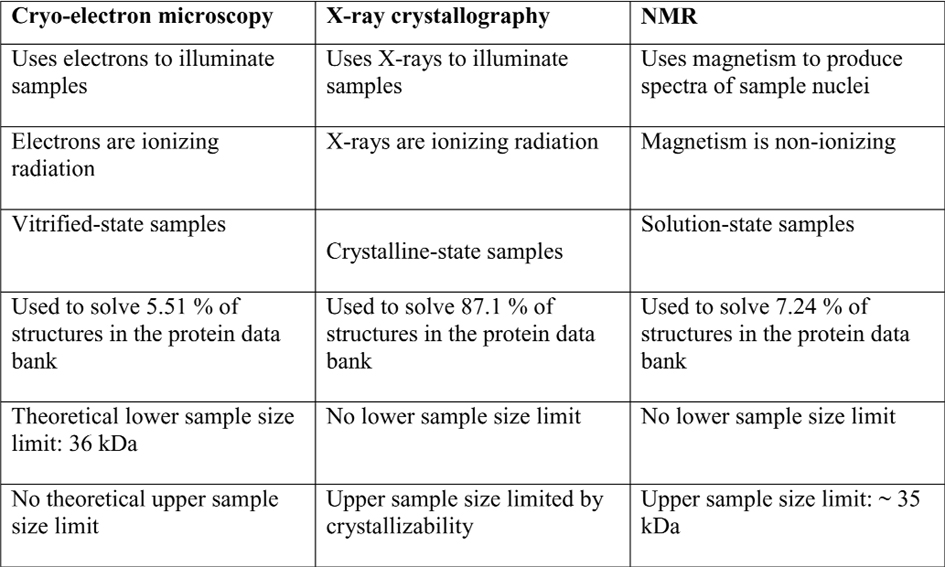
(The author is pursuing research in the area of structural biology in Poland)